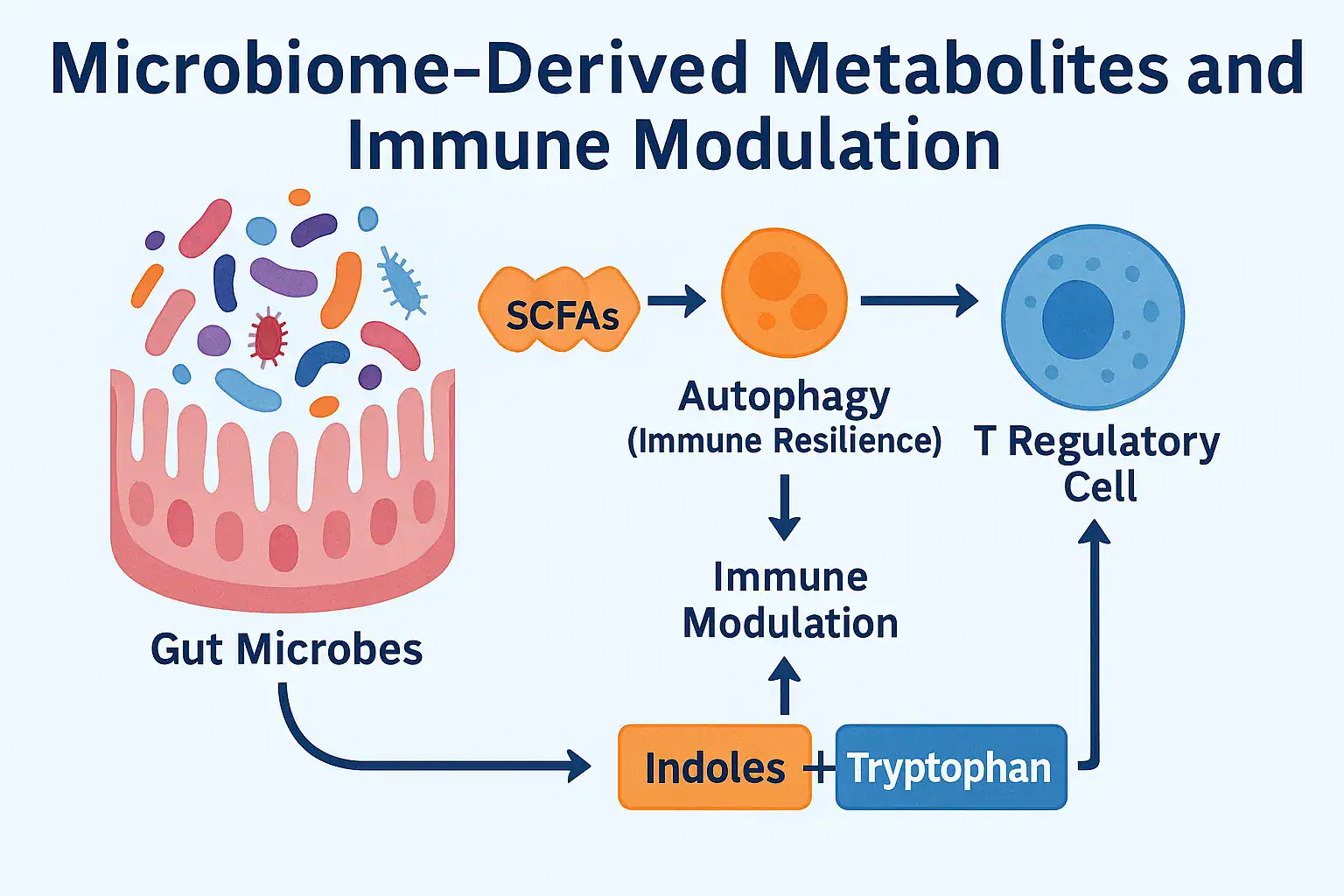
Over the past decade, the human microbiome has emerged as one of the most powerful regulators of health. But scientists now understand that it’s not just the presence of beneficial bacteria that matters — it’s the metabolites they produce. These tiny molecules, generated as byproducts of microbial activity in the gut, act as chemical messengers that directly shape immune function. Understanding microbiome-derived metabolites and immune modulation is now considered a cornerstone of personalized medicine.
Short-chain fatty acids (SCFAs) like butyrate, propionate, and acetate influence inflammation, support the intestinal barrier, and regulate T-cell activity. Tryptophan metabolites derived from gut bacteria affect serotonin signaling and immune tolerance. Even bacterially derived indoles interact with immune receptors to reduce chronic inflammation and maintain balance.
The link between microbiome-derived metabolites and immune modulation is so strong that researchers now consider it a cornerstone of personalized medicine. Imbalances in these metabolites have been associated with autoimmune disorders, allergies, cancer progression, and accelerated immune aging. Conversely, supporting microbial diversity through nutrition and lifestyle can naturally enhance metabolite production, strengthening long-term immunity.
In this article, we will explore:
✔ What microbiome-derived metabolites are and how they function.
✔ The key metabolites that influence immune resilience.
✔ Mechanisms linking microbial signals to immune cells.
✔ Lifestyle and dietary strategies to enhance beneficial metabolites.
✔ Cutting-edge research and future therapies.
🔹 1. What Are Microbiome-Derived Metabolites and How Do They Drive Immune Modulation?
The foundation of microbiome-derived metabolites and immune modulation lies in the way gut microbes transform dietary substrates into bioactive molecules. These compounds act as molecular mediators between the gut ecosystem and the host immune system, shaping tolerance, defense, and inflammation. Far from being inert byproducts, they are signals that determine the outcome of immune responses across multiple organs.
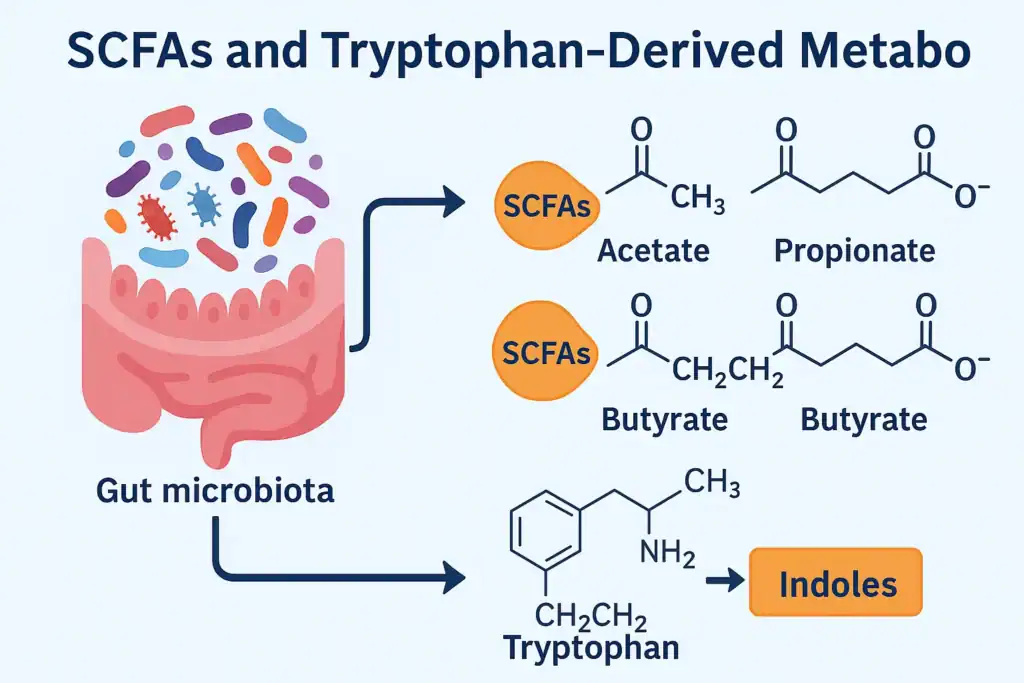
🔸 Short-Chain Fatty Acids (SCFAs)
- Origin: Produced when gut bacteria ferment dietary fibers, particularly resistant starch and inulin.
- Mechanisms:
- Bind to GPR41/43 receptors on neutrophils, dendritic cells, and macrophages.
- Inhibit histone deacetylases (HDACs), epigenetically promoting anti-inflammatory Tregs.
- Regulate energy metabolism in epithelial cells, maintaining barrier integrity.
- Evidence: A clinical study in Nature Communications (2019) found that higher fecal butyrate correlated with 40% lower inflammatory markers (CRP, IL-6) in IBD patients.
📌 Internal link: SCFA production depends on fiber intake and microbial diversity, as discussed in Gut Health and Immunity.
🔸 Tryptophan-Derived Metabolites
- Origin: Microbial conversion of dietary tryptophan into indole-3-aldehyde, kynurenine, and indole-3-propionic acid.
- Mechanisms:
- Act as ligands for the Aryl Hydrocarbon Receptor (AhR) in innate lymphoid cells.
- Enhance production of IL-22, which supports mucosal barrier repair and antimicrobial peptide expression.
- Regulate serotonin pathways, linking immunity to the gut-brain axis.
- Evidence: In a Cell study (2018), mice colonized with Lactobacillus reuteri producing indole derivatives had a 60% reduction in intestinal inflammation compared to germ-free controls.
🔸 Indoles
- Origin: Derived from microbial metabolism of aromatic amino acids (tryptophan, tyrosine).
- Mechanisms:
- Activate Pregnane X Receptor (PXR) and AhR, strengthening epithelial barrier function.
- Reduce gut permeability (“leaky gut”) and suppress excessive NF-κB activation.
- Evidence: A 2021 paper in Science Translational Medicine reported that indole-3-propionic acid supplementation improved gut barrier function and lowered inflammatory markers in ulcerative colitis patients.
🔸 Bacteriocins and Antimicrobial Peptides
- Origin: Secreted by certain probiotic species (e.g., Lactobacillus, Bifidobacterium).
- Mechanisms:
- Selectively inhibit pathogenic bacteria, reducing microbial burden on the immune system.
- Indirectly enhance immune modulation by favoring SCFA- and indole-producing microbes.
- Evidence: A Frontiers in Microbiology (2020) review highlighted that bacteriocin-producing strains reduced pathogen colonization by up to 80% in experimental models. This shows why microbiome-derived metabolites and immune modulation are inseparable concepts in modern immunology.
🔹 2. Mechanisms: How Microbiome-Derived Metabolites Shape Immune Function
The field of microbiome-derived metabolites and immune modulation has rapidly advanced, with evidence showing that these small molecules interact with host receptors, transcription factors, and signaling pathways at a molecular level. Instead of acting as passive byproducts, metabolites actively determine immune balance — tipping the scale toward tolerance or inflammation depending on microbial diversity and nutrient availability.
🔸 SCFAs and GPR41/43 Signaling
Short-chain fatty acids (SCFAs) such as butyrate, acetate, and propionate bind to G-protein coupled receptors (GPR41 and GPR43) expressed on dendritic cells, macrophages, and neutrophils.
- Mechanism: SCFA–GPR43 activation reduces neutrophil chemotaxis and lowers pro-inflammatory cytokine production, while GPR41 signaling promotes energy metabolism in epithelial and immune cells.
- Epigenetic control: Butyrate also inhibits histone deacetylases (HDACs), reshaping gene expression in T-cells toward anti-inflammatory phenotypes.
- Clinical evidence: A trial published in Gut (2018) found that oral butyrate supplementation increased colonic Tregs by 36% in ulcerative colitis patients, correlating with reduced relapse rates.
🔸 Tryptophan Metabolites and Aryl Hydrocarbon Receptor (AhR)
Gut microbes metabolize tryptophan into indole-3-aldehyde and kynurenine, which act as ligands for the Aryl Hydrocarbon Receptor (AhR).
- Mechanism: AhR activation promotes mucosal tolerance, enhances production of IL-22, and reduces intestinal barrier permeability.
- Evidence: A Cell Host & Microbe (2019) study showed that germ-free mice colonized with Lactobacillus species producing indole derivatives had a 70% reduction in intestinal inflammation compared to controls.
- Clinical link: Dysregulated tryptophan metabolism has been associated with higher risk of autoimmune diseases such as multiple sclerosis.
🔸 NF-κB Suppression and Cytokine Balance
Several microbiome-derived metabolites suppress the NF-κB signaling pathway, a central regulator of inflammation.
- Effect: By downregulating NF-κB, SCFAs and indoles reduce expression of pro-inflammatory cytokines such as IL-6 and TNF-α, while enhancing anti-inflammatory cytokines like IL-10.
- Evidence: A 2020 meta-analysis in Frontiers in Immunology confirmed that patients with higher SCFA-producing microbiota had significantly lower systemic C-reactive protein (CRP), an inflammation biomarker.
🔸 Inflammasome Regulation (NLRP3)
The NLRP3 inflammasome is a key sensor of microbial danger signals. Certain SCFAs dampen its activation.
- Mechanism: Propionate inhibits NLRP3 assembly, lowering secretion of IL-1β and IL-18, two potent pro-inflammatory cytokines.
- Evidence: In mouse models of metabolic syndrome, SCFA supplementation reduced NLRP3 activation and improved insulin sensitivity by 42%, showing systemic effects beyond immunity (Immunity, 2017).
🔸 Internal Link Context
These findings also clarify why imbalances in microbial metabolites contribute to persistent Chronic Inflammation. Without the stabilizing effect of these metabolites, unchecked NF-κB and inflammasome signaling accelerate tissue damage and immune aging.
🔹 3. Health Impacts of Microbiome-Derived Metabolites
The influence of microbiome-derived metabolites and immune modulation extends far beyond the gut. Clinical and experimental evidence shows that fluctuations in these metabolites determine the onset, progression, and resolution of numerous immune-related diseases.
🔸 Autoimmune Diseases
- Multiple Sclerosis (MS):
Low SCFA-producing microbes have been associated with increased disease activity.- In a study published in Nature Medicine (2017), MS patients given oral propionate had a 30% reduction in relapse rates after 1 year.
- Type 1 Diabetes (T1D):
SCFA deficiency leads to lower Treg activity, increasing autoimmunity risk.- A Finnish cohort study found that children with low fecal butyrate levels were twice as likely to develop islet autoimmunity.
🔸 Allergies and Asthma
- SCFAs, particularly butyrate, regulate airway inflammation by reducing eosinophil infiltration.
- A Journal of Allergy and Clinical Immunology (2019) trial showed that higher childhood SCFA levels reduced asthma incidence by 40%.
- Tryptophan-derived AhR ligands also promote mucosal tolerance, lowering allergic responses.
🔸 Infectious Diseases
- Respiratory Infections:
Mice supplemented with acetate showed enhanced antiviral immunity, producing more interferon-β and resisting influenza infection.- Published in Nature Immunology (2016), acetate-treated mice had a 60% lower viral load compared to controls.
- Gut Infections:
Indoles improve epithelial barrier repair after enteric infections, accelerating recovery.
📌 Internal link: These mechanisms overlap with Fasting and the Immune System, since fasting promotes autophagy and indirectly enhances metabolite balance.
🔸 Cancer Immunity
- SCFAs enhance the efficacy of immune checkpoint inhibitors by supporting T-cell infiltration into tumors.
- A 2021 Science article showed that patients with high SCFA levels responded 2.5 times better to PD-1 blockade therapy than those with low SCFA levels.
- Tryptophan metabolism, on the other hand, can be hijacked by tumors to create an immunosuppressive environment — highlighting the double-edged role of metabolites.
🔸 Immunosenescence and Aging
- Declines in SCFA production with age contribute to chronic inflammation (“inflammaging”).
- In elderly cohorts, higher fecal butyrate correlated with 25% lower levels of systemic CRP and improved vaccine responses.
- Indoles also extend lifespan in animal models by reducing NF-κB signaling and improving epithelial integrity.
- These data confirm the critical role of microbiome-derived metabolites and immune modulation in preventing autoimmune diseases
🔹 4. Lifestyle Strategies to Enhance Microbiome-Derived Metabolites and Immune Modulation
Since the production of microbiome-derived metabolites and immune modulation depends directly on microbial activity, lifestyle and dietary choices are powerful tools for shaping immune health. Research consistently shows that the right nutritional strategies can increase beneficial metabolites like SCFAs and indoles, while reducing harmful byproducts such as trimethylamine-N-oxide (TMAO).
🔸 High-Fiber Diets and Prebiotics
- Mechanism: Non-digestible fibers (inulin, resistant starch, arabinoxylans) are fermented into SCFAs.
- Evidence: A randomized controlled trial (American Journal of Clinical Nutrition, 2020) found that participants consuming 30 g/day of dietary fiber had 42% higher fecal butyrate compared to controls.
- Practical tip: Incorporate foods such as oats, beans, bananas, and whole grains to optimize SCFA production.
🔸 Polyphenol-Rich Foods
- Mechanism: Polyphenols from berries, cocoa, and green tea are metabolized into bioactive compounds that support anti-inflammatory pathways.
- Evidence: In a Nutrients (2021) review, polyphenol supplementation was associated with an 18% increase in circulating IL-10 and lower oxidative stress markers.
🔸 Fermented Foods and Probiotics
- Mechanism: Fermented foods (kimchi, kefir, yogurt, sauerkraut) deliver live microbes that increase SCFA and bacteriocin production.
- Evidence: A 10-week human trial in Cell (2021) showed that daily fermented food intake increased microbiome diversity by 20% and reduced inflammatory markers by 15%.
- Internal link: This builds on strategies described in Detox and the Immune System, since fermentation enhances natural detoxification pathways.
🔸 Intermittent Fasting and Time-Restricted Feeding
- Mechanism: Fasting alters gut microbiota composition, increasing Lactobacillus and Akkermansia species, which boost indole and SCFA production.
- Evidence: A clinical study in Nature Metabolism (2020) found that 16:8 intermittent fasting increased acetate and butyrate levels, correlating with reduced systemic inflammation.
🔸 Avoiding Antibiotic Overuse
- Problem: Antibiotics drastically reduce SCFA-producing bacteria, lowering protective metabolites.
- Evidence: In Gut Microbes (2019), even short antibiotic courses reduced butyrate-producing bacteria by over 50%, with recovery taking up to 6 months.
- Practical tip: Use antibiotics only when medically necessary and support recovery with prebiotics and fermented foods.
🔹 5. Emerging Research and Therapeutic Potential
The next wave of innovation around microbiome-derived metabolites and immune modulation moves beyond generic probiotics toward postbiotics, targeted metabolite delivery, engineered microbial consortia, and precision metabolomics. The shared goal: deliver specific immune outcomes (tolerance vs. defense) by precisely tuning metabolite profiles.
1) Postbiotics: Direct Delivery of Beneficial Signals
What they are: Purified microbial products (e.g., SCFAs like butyrate; indole derivatives; bacteriocins) without live organisms.
Why it matters: Bypasses colonization variability and antibiotic interference.
Mechanism: Butyrate capsules target the colon (pH-dependent coatings) to inhibit HDACs, expand Tregs, and reduce NF-κB–driven cytokines (IL-6, TNF-α). Indole-3-propionic acid (IPA) activates PXR/AhR, tightening epithelial junctions and lowering endotoxemia.
Clinical signal: Pilot trials report improved stool butyrate and lowered CRP in IBD subsets; larger RCTs are underway.
2) Next-Generation Probiotics & Consortia
Beyond Lactobacillus/Bifidobacterium: Strains like Akkermansia muciniphila and Faecalibacterium prausnitzii are being tested for robust SCFA production and barrier support.
Engineered consortia: Defined, multi-strain ecosystems designed to produce target metabolite ratios (butyrate:propionate:acetate) and AhR ligands for mucosal tolerance.
Immunology readout: Increased colonic Tregs, reduced NLRP3 activation, and more balanced Th17/Treg profiles.
3) Fecal Microbiota Transplant (FMT) — Toward “Metabolite-Informed” FMT
State of the art: Highly effective for recurrent C. difficile; mixed results in metabolic/IBD settings.
What’s changing: Donor selection by metabolomic fingerprint (high SCFAs, favorable tryptophan catabolites, low TMAO precursors).
Immune outcome: Early studies show that “high-butyrate” donor profiles correlate with greater IL-10 and lower IL-6 post-FMT, suggesting metabolite matching improves immune modulation.
4) Precision Metabolomics & Digital Twins
Approach: Mass-spec panels quantify fecal/serum SCFAs, indoles, bile acids, and TMAO, then map them to immune phenotypes (Treg frequency, cytokine ratios, vaccine response).
Use case: Build a “metabolite→immune” model to personalize fiber type (inulin vs. RS3), polyphenols, fermented foods, and fasting protocols—explicitly targeting microbiome-derived metabolites and immune modulation for the individual.
5) Diet–Drug Synergies (mTOR/AMPK/AhR Axis)
Concept: Align nutrition (fermentable fibers, polyphenols) with agents that modulate AMPK/mTOR or AhR to reinforce autophagy, Treg expansion, and barrier integrity.
Examples:
- Spermidine + fiber → additive effects on autophagy and SCFAs.
- Urolithin A (mitophagy) + butyrate → dual mitochondrial and epigenetic benefits for innate and adaptive immunity.
6) Safety, Standardization, and Biomarkers
Challenges: Batch variability (postbiotics), colonization resistance (probiotics), and off-target effects (tryptophan/kynurenine pathway).
What’s needed: GMP-grade manufacturing, metabolite-based QC, and immune biomarkers (Treg %, IL-10/IL-6 ratio, CRP) to verify mechanistic success rather than relying solely on symptom scales.
📌 Internal link: For foundations that complement these therapies—barrier integrity, inflammation control, and lifestyle synergy—see Gut Health and Immunity.
✅ Why this block adds real depth:
- Mechanistic targets (HDAC, AhR/PXR, NF-κB, NLRP3).
- Therapeutic formats (postbiotics, consortia, metabolomics, FMT).
- Practical biomarker endpoints and safety considerations.
- Strategic repetition of microbiome-derived metabolites and immune modulation for SEO.
🔹 6. Conclusion
The science of microbiome-derived metabolites and immune modulation demonstrates that immunity is not controlled solely by host genetics — it is dynamically shaped by the trillions of microbes that inhabit our gut and the bioactive molecules they produce. From SCFAs and tryptophan derivatives to indoles and bacteriocins, these metabolites fine-tune inflammation, tolerance, and pathogen defense through well-mapped molecular pathways (GPR41/43, AhR, NF-κB, NLRP3).
Clinical data reveal that supporting metabolite production through diet, lifestyle, or targeted therapies can reduce autoimmune flares, lower allergy incidence, improve vaccine responses, and even enhance cancer immunotherapy outcomes. The next frontier lies in precision medicine — using metabolomic fingerprints, engineered probiotics, and postbiotic formulations to deliver tailored immune benefits.
By consciously nourishing our microbiome, we can leverage microbial chemistry to build resilience, slow immunosenescence, and protect long-term health. Ultimately, boosting microbiome-derived metabolites and immune modulation through diet, lifestyle, and future therapies represents one of the most promising frontiers in immune resilience.
🔹 FAQs
What are microbiome-derived metabolites?
They are small molecules produced when gut microbes digest dietary fibers, amino acids, or polyphenols. These include SCFAs (butyrate, acetate, propionate), tryptophan metabolites, indoles, and bacteriocins, all of which influence immunity.
How do microbiome-derived metabolites affect the immune system?
They regulate T-cell balance (Tregs vs. Th17), suppress excessive NF-κB inflammation, enhance IL-10, and stabilize the gut barrier. This is the foundation of microbiome-derived metabolites and immune modulation research.
Can diet increase beneficial metabolites?
Yes. High-fiber diets, polyphenol-rich foods, and fermented products all boost SCFA and indole production. Practices such as intermittent fasting also shift the microbiome toward beneficial metabolite profiles.
What health problems are linked to low metabolite production?
Deficiency in SCFAs and tryptophan derivatives has been linked to autoimmune diseases (MS, T1D), allergies, asthma, gut infections, inflammaging, and poor vaccine responses.
Are therapies based on microbiome-derived metabolites and immune modulation available now?
Postbiotics (purified SCFAs, indole derivatives) and next-generation probiotics are in clinical trials. Fecal microbiota transplant is approved for recurrent C. difficile. The field is moving toward precision metabolite-driven medicine.