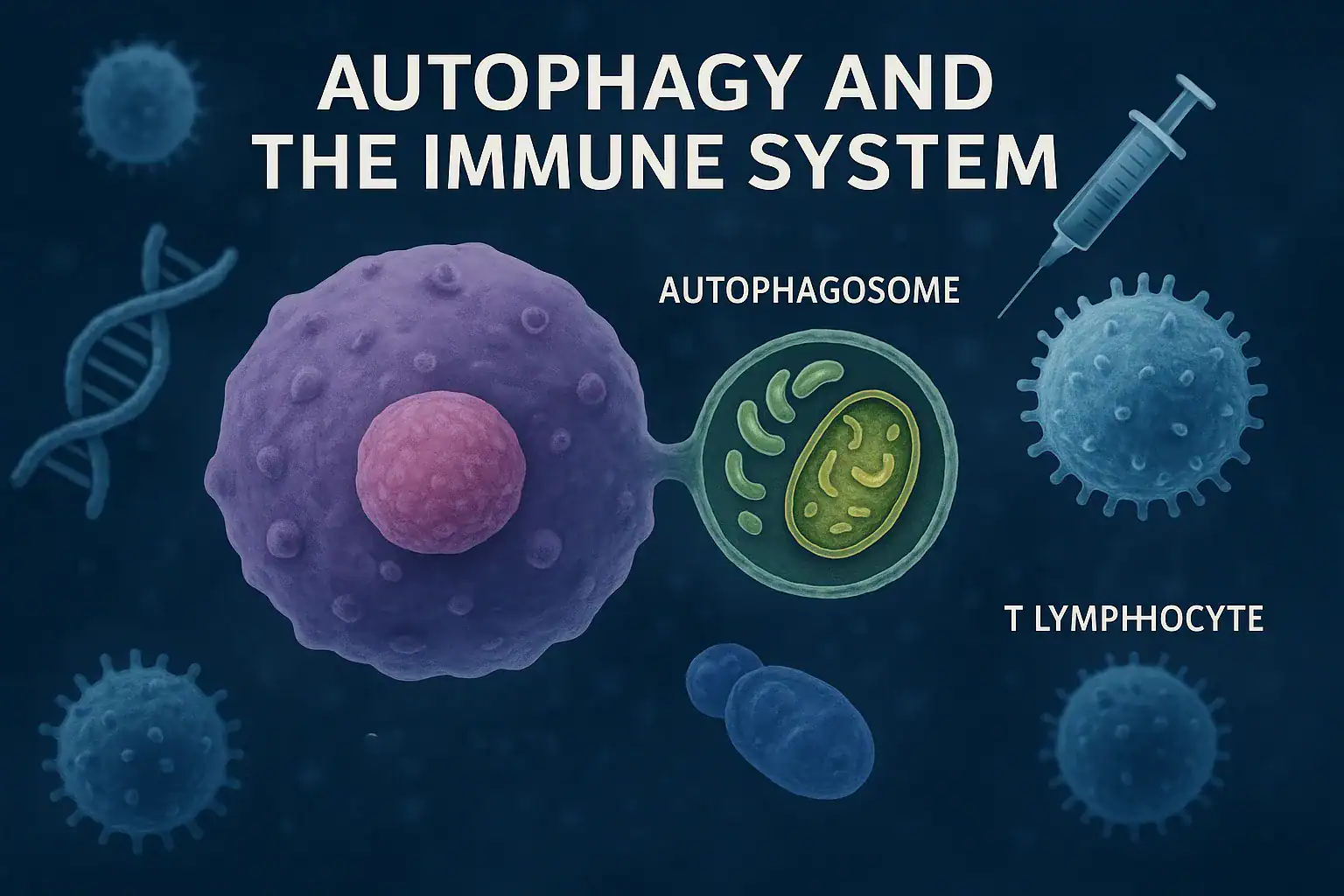
Have you ever wondered how your body gets rid of old, damaged, or “junk” cells that no longer work properly? The answer lies in a fascinating biological process called autophagy—literally meaning “self-eating.” Far from being destructive, this cellular recycling system is essential for maintaining health, energy, and especially strong immunity.
Scientists now recognize that the connection between autophagy and the immune system is one of the most important discoveries in modern biology. Understanding autophagy and the immune system is essential not only for longevity but also for everyday infection resistance. By breaking down dysfunctional cell parts and reusing their components, autophagy not only prevents toxic buildup but also empowers immune cells to fight infections, control inflammation, and delay the effects of aging.
In fact, impaired autophagy has been linked to conditions such as chronic inflammation, neurodegeneration, cancer, and accelerated immune aging—sometimes called immunosenescence. On the other hand, lifestyle habits such as intermittent fasting, quality sleep, and nutrient-rich diets have been shown to activate autophagy naturally, strengthening defenses from the inside out.
In this article, we’ll explore:
✔ What autophagy is and how it works.
✔ How autophagy supports the immune system at every level.
✔ The role of autophagy in aging and disease prevention.
✔ Practical, science-backed ways to boost autophagy naturally.
🔹 1. What Is Autophagy and How Does It Support the Immune System?
At its core, autophagy is the body’s built-in recycling program. The word comes from the Greek auto (“self”) and phagy (“eating”), meaning “self-eating.” But instead of harming the body, autophagy is a life-sustaining process. This shows that the link between autophagy and the immune system is central to cellular defense.
Here’s how it works: when a cell detects damaged proteins, dysfunctional mitochondria, or invading pathogens, it forms a double-layered membrane around them. This structure, called an autophagosome, delivers the unwanted material to the lysosome, where it is broken down into raw components. These components are then reused to build new proteins and organelles, keeping cells efficient and resilient.
Why Autophagy Matters for Immunity
- It eliminates intracellular pathogens, including viruses and bacteria, before they can spread.
- It removes damaged immune cells, preventing dysfunction.
- It provides energy and building blocks for rapidly dividing immune cells during infection.
📌 Scientific evidence: Studies in Nature Reviews Immunology highlight that autophagy is not only a housekeeping mechanism but also a frontline immune defense, bridging innate and adaptive immunity.
📌 Internal link: Autophagy is also a key reason why fasting improves health outcomes—see Fasting and the Immune System.
🔹 2. Autophagy and the Immune System
The relationship between autophagy and the immune system is far more than maintenance—it is a survival mechanism. Without proper autophagy, immune defenses weaken, inflammation rises, and susceptibility to infections increases.
Researchers now agree that boosting autophagy and the immune system together could be a key anti-aging strategy.
🔸 Autophagy in T-Cells and Adaptive Immunity
T-cells are the “commanders” of the adaptive immune system, orchestrating responses against viruses and tumors. Studies show that autophagy maintains T-cell survival and energy supply. When autophagy is impaired, T-cells lose function and die prematurely.
📌 Scientific evidence: A 2016 study in Cell Metabolism found that deleting autophagy-related genes (ATG7) in mice caused rapid T-cell decline and reduced ability to fight viral infections.
🔸 Autophagy in Natural Killer (NK) Cells
NK cells are part of the innate immune system, responsible for detecting and destroying infected or cancerous cells. Autophagy boosts their cytotoxic activity by ensuring mitochondria remain healthy, providing the energy required for rapid immune attacks.
📌 Scientific evidence: Research in Frontiers in Immunology (2020) showed that enhancing autophagy pathways improved NK cell function against tumors, suggesting therapeutic potential.
🔸 Autophagy as an Antiviral Shield
Viruses often try to “hijack” the cell’s machinery to replicate. Autophagy can intercept and degrade viral particles before replication is complete, a process known as xenophagy.
📌 Scientific evidence: A study published in Nature Microbiology demonstrated that autophagy restricted replication of influenza A virus by targeting viral ribonucleoproteins for degradation.
🔸 Balancing Inflammation
Chronic inflammation damages tissues and accelerates immune aging. Autophagy acts like a “brake,” clearing inflammatory signals and preventing immune overreaction.
📌 Scientific evidence: Research in Science Translational Medicine revealed that defective autophagy in macrophages leads to uncontrolled inflammation, contributing to diseases such as Crohn’s and rheumatoid arthritis.
🔹 3. Autophagy, Aging, and Immunosenescence
As we age, the efficiency of autophagy declines, and this directly weakens autophagy and the immune system, accelerating immunosenescence. The progressive weakening of the immune system that leaves older adults more vulnerable to infections, cancer, and chronic inflammation.
🔸 Decline of Autophagy with Age
Cells from elderly individuals often show fewer autophagosomes and reduced activity of autophagy-related genes (ATGs). Without this recycling process, damaged proteins and mitochondria accumulate, leading to energy deficits and immune dysfunction.
📌 Scientific evidence: A study in Aging Cell (2019) demonstrated that impaired autophagy accelerates thymic atrophy and reduces the production of naïve T-cells, a hallmark of immune aging.
🔸 Inflammaging and Autophagy Deficiency
When autophagy slows, cells release pro-inflammatory molecules such as IL-6 and TNF-α, fueling inflammaging—the chronic, low-grade inflammation associated with aging. This inflammation further disrupts immune function, creating a vicious cycle.
📌 Scientific evidence: Research in Nature Reviews Molecular Cell Biology confirmed that defective autophagy is one of the biological roots of inflammaging.
🔸 Protective Role of Enhanced Autophagy
On the other hand, interventions that stimulate autophagy—such as caloric restriction, intermittent fasting, or pharmacological compounds like rapamycin—have been shown to restore aspects of immune youthfulness.
📌 Scientific evidence: A landmark trial in Nature Aging reported that low-dose rapamycin improved vaccine responses in older adults by enhancing autophagy and reducing immune exhaustion.
📌 Internal link: For a deeper dive into how the immune system ages and how to slow it, see Immunosenescence: Why the Immune System Ages and How to Slow It Down.
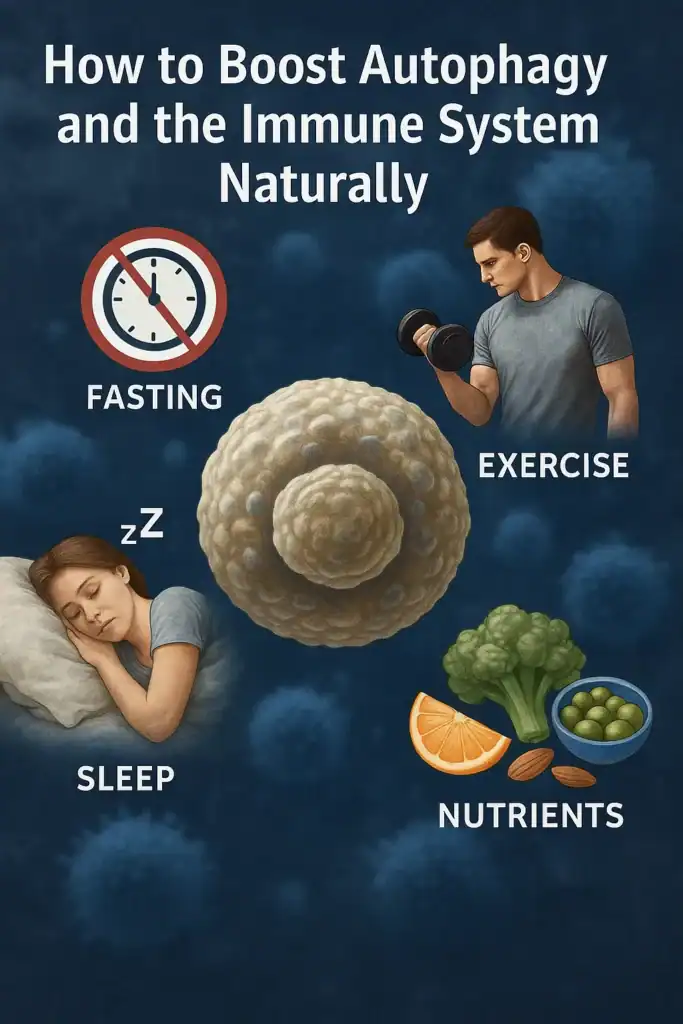
🔹 4. How to Boost Autophagy Naturally
While scientists are studying drugs to activate autophagy, the most effective and accessible ways to enhance this process come from daily lifestyle choices. These strategies help keep the connection between autophagy and the immune system strong throughout life.
🔸 Intermittent Fasting
Fasting is one of the most powerful natural triggers of autophagy. When nutrient intake drops, cells switch from growth mode to repair mode, recycling damaged proteins and improving immune resilience.
📌 Scientific evidence: A study in Cell Stem Cell (2014) showed that prolonged fasting cycles stimulated autophagy, regenerated immune cells, and reduced immunosuppression.
📌 Internal link: For a full guide on how fasting enhances immunity, see Fasting and the Immune System.
🔸 Exercise
Moderate physical activity activates autophagy in muscle and immune cells, improving mitochondrial health and boosting natural killer (NK) cell function. Overtraining, however, can suppress immunity.
📌 Scientific evidence: Research in Autophagy Journal (2017) confirmed that endurance exercise induces autophagy in both skeletal muscle and lymphocytes, strengthening immune performance.
🔸 Sleep and Circadian Rhythm
Deep sleep is critical for activating cellular repair pathways, including autophagy. Disrupted circadian rhythms reduce autophagic activity, impairing immune balance.
📌 Scientific evidence: Studies in Nature Communications (2020) showed that circadian disruption suppresses autophagy and weakens antiviral immunity.
🔸 Nutrients and Phytochemicals
Certain natural compounds have been found to stimulate autophagy, including:
- Resveratrol (found in grapes and red wine)
- Curcumin (from turmeric)
- EGCG (green tea)
- Omega-3 fatty acids
📌 Scientific evidence: A review in Frontiers in Pharmacology (2019) highlighted these compounds as safe autophagy enhancers with immune-protective effects.
📌 Internal link: Supporting detoxification also improves nutrient absorption and immune resilience—see Detox and the Immune System.
🔹 5. Health Risks When Autophagy and the Immune System Fail
When autophagy is defective, the body loses its ability to recycle damaged proteins, dysfunctional organelles, and intracellular pathogens. This failure doesn’t just accelerate aging—it lays the foundation for some of the most serious chronic diseases.
🔸 Neurodegenerative Disorders
Without autophagy, abnormal proteins accumulate in neurons, forming toxic aggregates.
- Alzheimer’s disease: β-amyloid plaques and tau tangles result partly from defective autophagic clearance.
- Parkinson’s disease: Autophagy failure prevents removal of misfolded α-synuclein, leading to neuronal death.
📌 Scientific evidence: A study in Nature Neuroscience (2018) revealed that impaired lysosomal-autophagy function accelerated β-amyloid buildup and cognitive decline in animal models.
🔸 Cancer Development
Autophagy plays a dual role in cancer:
- In healthy cells, it prevents tumorigenesis by removing damaged DNA and oncogenic proteins.
- In advanced cancers, however, tumor cells hijack autophagy to fuel their growth under stress.
📌 Scientific evidence: A Cell (2016) review showed that defective autophagy in immune cells reduces tumor surveillance, while excessive autophagy in cancer cells increases their survival.
🔸 Chronic Inflammation and Autoimmune Diseases
Autophagy normally suppresses uncontrolled inflammation by clearing defective mitochondria (mitophagy) and reducing the release of inflammatory molecules such as IL-1β.
- In Crohn’s disease, mutations in the ATG16L1 gene disrupt autophagy and increase gut inflammation.
- In rheumatoid arthritis, defective autophagy in macrophages amplifies inflammatory cytokine release.
📌 Scientific evidence: Research in Science Translational Medicine (2016) confirmed that ATG16L1 mutations impair autophagy in intestinal cells, directly linking defective autophagy to Crohn’s pathogenesis.
🔸 Immune Aging (Immunosenescence)
Defective autophagy accelerates immunosenescence by reducing T-cell diversity and promoting inflammaging. This not only increases vulnerability to infections but also decreases vaccine effectiveness in older adults.
📌 Internal link: See Immunosenescence: Why the Immune System Ages and How to Slow It Down for a deeper look at this connection.
🔸 Metabolic Disorders
Autophagy failure also contributes to metabolic diseases:
- Type 2 diabetes: Impaired autophagy in pancreatic β-cells reduces insulin secretion.
- Obesity: Dysfunctional autophagy increases lipid accumulation and systemic inflammation.
📌 Scientific evidence: A Journal of Clinical Investigation (2019) study showed that autophagy deficiency in β-cells triggered insulin resistance and accelerated diabetes progression.
This highlights what happens when autophagy and the immune system break down together—disease risk rises dramatically.
🔹 6. Cutting-Edge Research on Autophagy
Modern science has moved from seeing autophagy as housekeeping to recognizing autophagy and the immune system as therapeutic targets, for aging and immune health. Here are some of the most promising areas of research on autophagy and the immune system.
🔸 mTOR Inhibition: Rapamycin and Rapalogs
Rapamycin inhibits the mTORC1 pathway, which normally suppresses autophagy. By lifting this brake, rapamycin enhances autophagic flux and improves immune resilience.
- Immune relevance: In older adults, low-dose rapamycin improved vaccine responses and reduced markers of immunosenescence.
- Caution: High doses may impair metabolism by affecting mTORC2, so clinical supervision is required.
📌 Nature Aging (2020) reported improved antibody responses to influenza vaccination in elderly participants treated with rapamycin derivatives.
🔸 AMPK Activation and Caloric-Restriction Mimetics
Drugs and natural compounds that activate AMPK or mimic caloric restriction can stimulate autophagy.
- Metformin: Activates AMPK, reduces chronic inflammation, and may improve infection outcomes in diabetic patients.
- Spermidine: Enhances protein clearance and has been linked to better cognition and immune resilience in older adults.
- Resveratrol: Works via SIRT1/AMPK to promote autophagy and mitochondrial quality control.
📌 Frontiers in Pharmacology (2019) reviewed spermidine as a natural autophagy inducer with potential for healthy aging.
🔸 Mitophagy Boosters: Urolithin A and NAD⁺
- Urolithin A: Derived from gut microbiota, promotes mitophagy (clearance of defective mitochondria), improving cellular energy in muscle and immune cells. Early trials show improved mitochondrial health in humans.
- NAD⁺ precursors (NR, NMN): Boost SIRT1 activity, supporting autophagy and immune regulation, though human evidence is still emerging.
🔸 TFEB Activation and Lysosomal Biogenesis
TFEB is a master regulator of lysosomal and autophagy-related genes. By stimulating TFEB, researchers aim to expand autophagic capacity, improving immune balance and resilience.
🔸 Senolytics and Inflammaging Control
Senescent cells secrete inflammatory molecules (the SASP) that accelerate immune aging. Senolytic drugs such as dasatinib + quercetin selectively remove these “zombie cells,” while also restoring healthy autophagy levels.
📌 Animal studies show senolytic therapy reduces systemic inflammation and improves infection resistance.
🔸 Lifestyle as a Natural Autophagy Trigger
- Exercise: Endurance and resistance training induce autophagy in muscle and lymphocytes, improving immune surveillance.
- Fasting: Time-restricted eating and intermittent fasting synchronize AMPK and SIRT1 pathways, enhancing autophagy and lowering inflammatory cytokines.
📌 Internal link: See Fasting and the Immune System for practical strategies to apply this science.
⚠️ Clinical Translation and Safety
- Balance is key: Too little autophagy leads to toxic buildup and inflammation, while too much can impair repair and growth.
- Medical supervision: Rapamycin, metformin, and senolytics should only be used under professional guidance.
- Best strategy today: Combine lifestyle interventions (sleep, fasting, exercise, nutrition) with medical oversight for advanced therapies.
🔹 7. Conclusion & Future Outlook
Autophagy and the immune system together form a cornerstone of long-term immune resilience.. By keeping cells clean, mitochondria efficient, and immune defenses sharp, autophagy provides a natural shield against infections, cancer, and chronic inflammation.
As research advances, therapies like rapamycin, spermidine, and senolytics may one day complement lifestyle strategies to fully harness the power of autophagy and the immune system. Until then, fasting, exercise, sleep optimization, and nutrient-rich diets remain the safest and most effective ways to keep this ancient survival program active.
📌 Internal resources:
- Fasting and the Immune System
- Detox and the Immune System
- Immunosenescence: Why the Immune System Ages and How to Slow It Down
📌 External authority:
For further reading, see this review in Nature Reviews Immunology: Autophagy and immunity: insights from human disease.
🔹 FAQs on Autophagy and the Immune System
What is the role of autophagy in the immune system?
Autophagy helps immune cells eliminate pathogens, recycle energy, and control inflammation. Without it, immunity weakens and chronic diseases accelerate.
Can boosting autophagy slow aging?
Yes. Studies show that enhanced autophagy delays immunosenescence, reduces chronic inflammation, and improves vaccine responses in older adults.
How can I naturally activate autophagy?
Time-restricted eating, intermittent fasting, exercise, and good sleep are proven ways to stimulate autophagy. Nutrients like spermidine, resveratrol, and omega-3s may also help. See Fasting and the Immune System for practical methods.
What happens when autophagy is impaired?
Impaired autophagy is linked to Alzheimer’s, Parkinson’s, cancer, diabetes, and autoimmune diseases like Crohn’s. It also accelerates immune aging and chronic inflammation.
Is it safe to use drugs like rapamycin to enhance autophagy?
Early studies are promising, but drugs like rapamycin and senolytics should only be taken under medical supervision. Lifestyle interventions remain the safest way to activate autophagy.